Acetone Polar or Nonpolar Solvent
The active site is the double bond. Web Lipids as a class of compounds are insoluble in water but are soluble in other organic solventsExamples of such solvents include acetone and ether.
Polar Vs Nonpolar Solvents Identifications And Examples Psiberg
Web That is solutes typically will dissolve best in solvents that have the most molecular similarities.

. Vapours rising from the boiling liquid are lighter than air. For example ethene readily undergoes additional reactions. Web The most common three types of solvents in organic chemistry are apolar polar aprotic and polar protic.
Web It is a nonpolar molecule. Web Many polymeric materials having chain-like structures similar to polyethylene are known. The structure of the fatty acids determines.
Alkanes-only single bonds 2. Polar bonds between C and more electronegative atoms O and N 2. Combine two functional groups ester alcohol acid.
An important polar aprotic solvent that dissolves both polar and nonpolar compounds Dioxane. It dissolves a wide range of both polar and nonpolar soils and is often used to dissolve and remove light oils fingerprints cutting fluids flux residues carbon deposits and mold release. Web an organosulfur compound.
Alcohol aldehyde ketone carboxyl amine organophosphate sulfhydral 1. Classified as an ether Ethanol. Fats have glycerol in addition to three fatty acids.
It is insoluble in polar solvents like water. Web Isopropyl alcohol CAS 67-63-0 is also referred to as IPA isopropanol 2-propanol and even rubbing alcohol more on that later. A powerful psychoactive drug.
Additionally solutes will be more soluble if the molecules in the solute are smaller than the ones in the solvent. Alkenes- CC double bonds 3. A heterocyclic organic compound.
Used in alcoholic beverages in thermometers as a solvent and as a fuel Fehlings reagent. Polymers formed by a straightforward linking together of monomer units with no loss or gain of material are called addition polymers or chain-growth polymersA listing of some important addition polymers and their monomer precursors is presented in the following. The polarity differentiation is done based on the dielectric constant of the solvents.
Polar solutes will dissolve better in polar solvents and nonpolar solutes will dissolve better in nonpolar solvents. It is soluble in non-polar solvents. Waxes steroids phospholipids and fats are the most common types of lipid groups.
Apolar or nonpolar solvents have low dielectric constants which refers to the electric charge between the molecules being evenly distributed. Aromatic-cyclic based on benzene B.

Is C3h6o Acetone Polar Or Non Polar Youtube
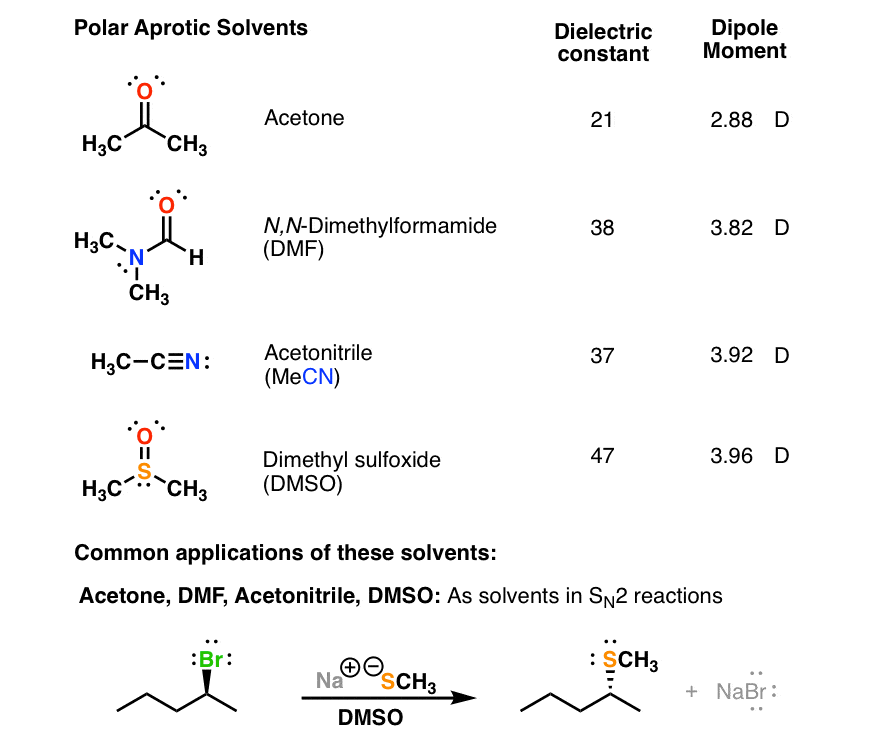
Polar Protic Polar Aprotic Nonpolar All About Solvents

Why Is Acetone A Good Solvent Properties Explanation Video Lesson Transcript Study Com
What Makes Acetone A Really Good Solvent What Allows It To Dissolve Both Polar And Non Polar Molecules Quora
No comments for "Acetone Polar or Nonpolar Solvent"
Post a Comment